Key Takeaways
- Electrical safety testing relies on a combination of an electrical safety tester and appropriate test equipment, including hipot, ground bond, insulation resistance, and leakage current measurement.
- Products must be evaluated against applicable national and international EMC and safety standards; early pre-compliance testing helps reduce development risk and redesign cycles.
- Electrical safety testing for medical devices involves stricter limits, clearly defined test conditions, and comprehensive documentation aligned with applicable standards.
- Combining EMC emissions and immunity testing with electrical safety checks helps identify potential issues earlier in the certification process.
- Clear test plans, calibrated equipment, and traceable reports support repeatable and auditable electrical safety testing from prototype through production.
Electrical safety and EMC compliance are closely linked aspects of product approval. Whether developing patient monitors, laboratory analyzers, or industrial control equipment, both must be addressed together. This guide explains how an EMC laboratory approaches electrical safety testing, which equipment is typically used, how medical device requirements differ from general industry practice, and how structured testing supports regulatory submissions.

What Electrical Safety Tests Include
Electrical safety testing typically covers a defined set of evaluations designed to assess protection against electric shock and insulation failure. These tests are performed using calibrated electrical safety test equipment under worst-case operating conditions.
- Hipot / Dielectric Strength: Verifies insulation integrity between mains circuits and accessible conductive parts.
- Ground Bond / Protective Earth Continuity: Confirms low-impedance protective earth paths capable of carrying fault currents.
- Insulation Resistance: Assesses long-term insulation performance under elevated voltage conditions.
- Leakage / Touch Current: Measures current that could flow through a user or patient during normal operation or fault conditions.
Each test is documented with defined limits, test configurations, and measured results.
Medical Device Electrical Safety Testing
Electrical safety testing for medical devices is subject to more stringent requirements due to patient-applied parts and potential patient exposure. Depending on classification (Type B, BF, or CF), leakage current limits and isolation requirements differ significantly.
Medical device electrical safety testing typically evaluates devices under:
- Normal Condition (NC)
- Single Fault Condition (SFC)
Testing covers power circuits, signal paths, enclosures, and applied parts. In addition to electrical measurements, labeling, markings, and instructions for use are reviewed for consistency with test results and applicable standards. The resulting documentation forms part of the technical file required for conformity assessment and certification by the responsible conformity assessment body.
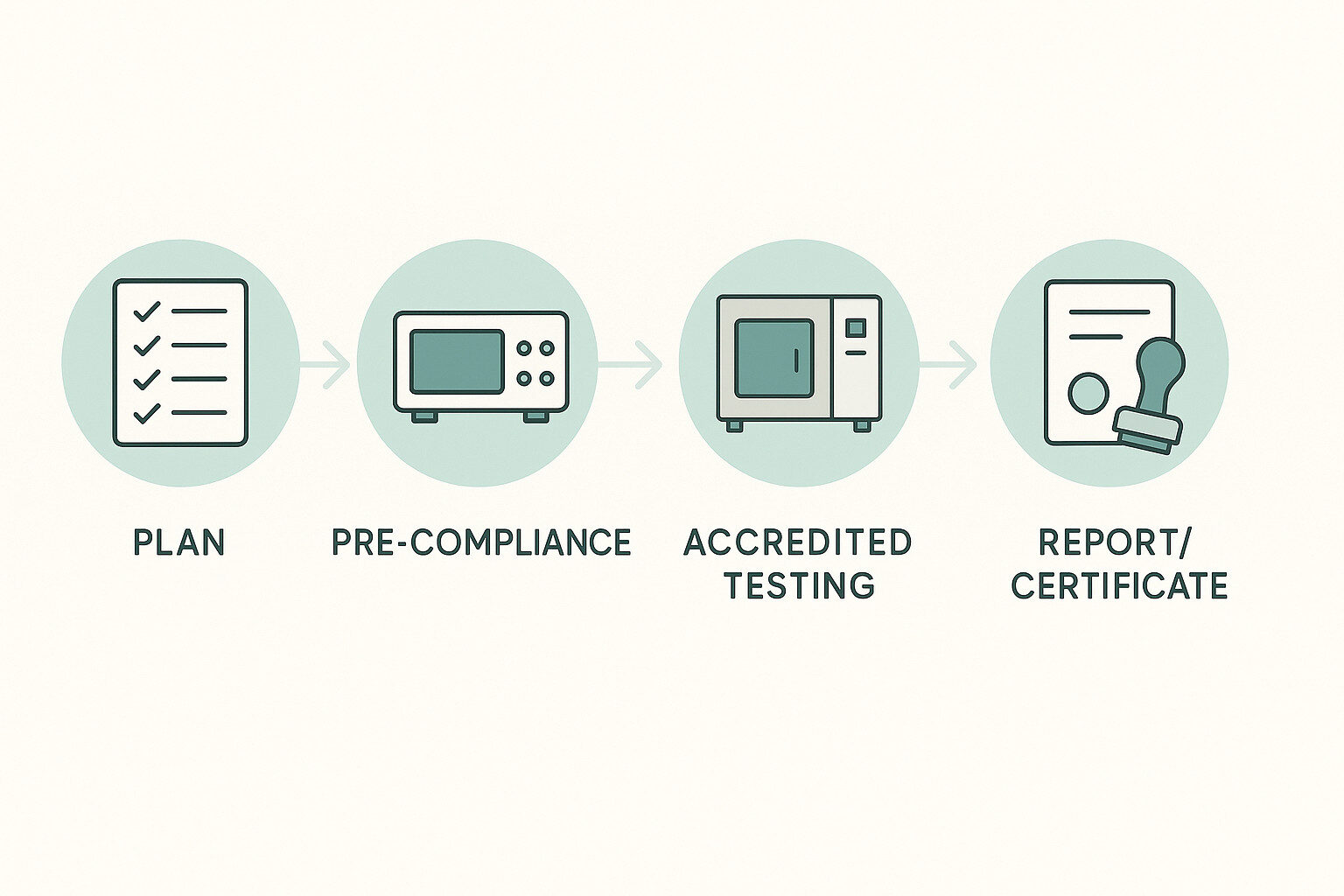
From Pre-Compliance to Formal Testing: A Practical Workflow
- Planning: Identify target markets, applicable EMC and safety standards, and define relevant operating modes for the device under test (DUT).
- Pre-Compliance Testing: Use laboratory equipment and fixtures to assess NC and SFC behavior early and reduce risk before formal testing.
- Formal Testing: Conduct electrical safety tests within an accredited testing program, using defined procedures and recorded evidence.
- Reporting & Documentation: Compile test reports and supporting documentation for regulatory and certification processes.
Equipment, Fixtures & Data Integrity
Reliable electrical safety testing depends on suitable equipment and controlled test setups. Typical elements include guarded test leads, Kelvin connections for ground bond testing, patient simulators, and automated test sequences.
All measurement results must be traceable to valid calibration certificates. Clear documentation and data traceability support audits and help streamline approvals across multiple regions.
Standards Landscape: EMC and Electrical Safety
Compliance programs generally reference both EMC and electrical safety standards. Common examples include:
- EMC: CISPR and IEC 61000 series, FCC requirements, CE conformity
- Product Safety: IEC 60601 (medical devices), IEC 61010 (laboratory equipment), IEC 62368-1 (audio/video and ICT equipment)
Addressing both EMC and electrical safety ensures devices operate reliably in their intended environments while maintaining protection against electrical hazards under normal and fault conditions.

FAQ
Q1. What does “tested for electrical safety” mean?
It indicates that the device has undergone defined electrical safety tests—such as hipot, ground bond, insulation resistance, and leakage current—with documented and traceable results.
Q2. How does electrical safety relate to EMC?
EMC focuses on emissions and immunity, while electrical safety addresses protection against shock and fire hazards. Most markets require compliance with both.
Q3. Are “electrical safety testing for medical devices” and “medical device electrical safety testing” different?
No. Both terms describe the same testing activities performed according to medical device safety standards and documented accordingly.
Q4. What helps avoid delays during certification?
Early pre-compliance testing, clearly defined operating modes, calibrated equipment, and complete labeling and documentation reviews.
Q5. Can EMC and electrical safety testing be scheduled together?
In many cases, yes. Coordinated testing can reduce overall project timelines and allow issues to be addressed within a single development cycle.
TPS Elektronik supports EMC laboratory workflows that integrate electrical safety testing with emissions and immunity assessments. We assist manufacturers in planning test programs, performing pre-compliance evaluations, and preparing documentation aligned with applicable national and international standards to support regulatory and certification processes.