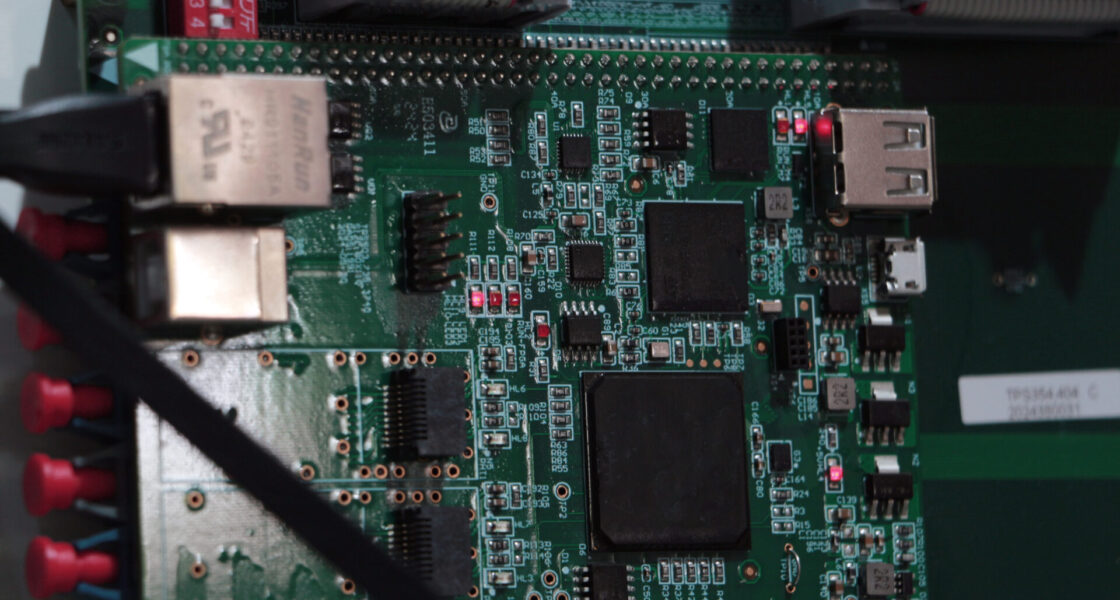
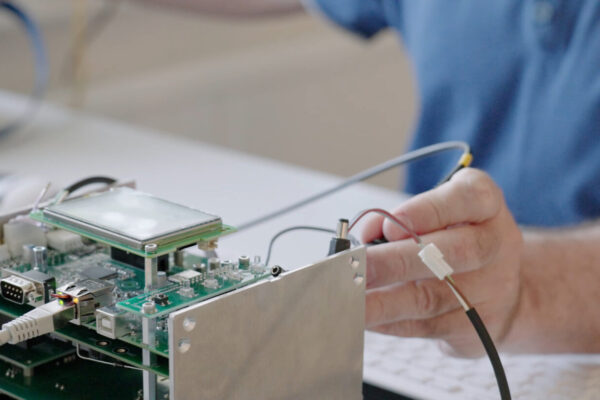
Initial Situation
A healthcare-technology company approached TPS Elektronik to modernize a critical diagnostic device. The existing PCB was outdated, no longer supported by the previous supplier, and lacked sufficient documentation.
After working with several software-only providers, the client realized they needed a partner capable of bridging PCB hardware and medical-device software — ensuring hardware reliability and software compatibility within one integrated process.
Challenge
- Outdated hardware platform and incomplete design documentation
- Need for a new PCB architecture and layout matched to medical requirements
- Tight integration between embedded hardware, drivers, and communication stacks
- Transparent budgeting and manufacturability from prototype to pilot series
- Coordination with international manufacturing partners
The TPS Elektronik Solution
1. PCB Redesign & Layout
- Developed a new circuit architecture and optimized multilayer layout (signal integrity, EMC, and thermal management).
- Created complete documentation to support conformity assessment and long-term maintenance.
- Conducted reliability, DFT, and DFM reviews to ensure consistent manufacturability.
2. End-to-End Manufacturing Support
- Organized rapid prototyping and pilot runs with suppliers matched to the required PCB stack-up.
- Coordinated with global manufacturing partners to balance cost, lead time, and scalability.
3. Embedded Hardware & Software Integration
- Implemented hardware drivers and communication interfaces aligned with the client’s software structure.
- Collaborated with the client’s software development team to ensure stable interfaces and secure data handling.
4. Tools, Verification & Consulting
- Utilized professional ECAD toolchains and automated checks (DRC/ERC, impedance control, BOM/version control).
- Advised on network topology and hardware partitioning for optimal system performance.
- Supported the client during documentation preparation for conformity assessment.
Results
- Regulatory readiness: The redesigned PCB and documentation supported the client’s conformity assessment activities.
- Improved reliability: The optimized layout reduced noise susceptibility and improved system stability in internal testing.
- Cost transparency: Early DFM/DFT reviews and multi-source planning improved predictability of development costs and lead times.
- Faster project delivery: Integrated design-to-pilot workflows shortened internal time-to-market compared to the previous product generation (per the client’s tracking).
Key Takeaway
By combining PCB redesign, embedded development, and manufacturing coordination, TPS Elektronik delivered a cohesive hardware-software foundation for a modernized diagnostic device.
The result: a compliant, maintainable platform ready for future product generations.
Next Steps
If you plan to modernize a regulated device or align your PCB with embedded requirements, TPS Elektronik will be glad to provide initial guidance and discuss suitable solutions.
Learn more about our PCB Design and related engineering services.